One can find classical electromagnetic solutions with the electron orbiting the proton but they are unstable as the smallest perturbation a radiation is a perturbation destroys them and starts the spiral. A rock rollingtumbling downhill is definitely unstable.
How Do Atoms Attain Stability By Forming Chemical Bonds Quora
They change spontaneously decay into other nuclei that are either in or closer to the band of stability.

. Find the staff directory and look up lisa goff. Same for water flowing downhill So why would an unstable atom become more stable. When this ratio is either too large or a.
Answer 1 of 1. 1 Answer David Drayer Truong-Son N. Alright so this question is on my biology homework.
These nuclear decay reactions convert one unstable isotope or radioisotope into another more stable isotope. Refer to periodic tableperiod 1 will gain 1 electronperiod 2 will gain 2 electronperiod 13 will gain 3 electronsperiod 14 will gain or lose 4 electronsperiod 15 will lose 3period 16 will. This activity is intended for middle and high school students.
Most naturally occurring atoms are stable and do not decay. 23 23 too small the nucleus of an atom becomes unstable b. Atoms can have at most 8 electrons in their outermost energy level.
When an atom has extra neutrons or protons it causes the element to become unstable. The body of the chicken is supported from above by the hips and acts as a pendulum between the hips. Find out in this clip.
The more you know. The chapter on atoms molecules and ions introduced the basic idea of nuclear structure that the nucleus of an atom is composed of protons and with the exception of 1 1H 1 1 H neutrons. The center of gravity of a chicken is below the hip joints.
So when does it become stable. Unstable atoms like your alkaline metals correct me if Im wrong gain stability by finishing off their valence electron count in the outer. One is the radioactive stability that involves forces between the particles that make up the nucleus and the other is the chemical.
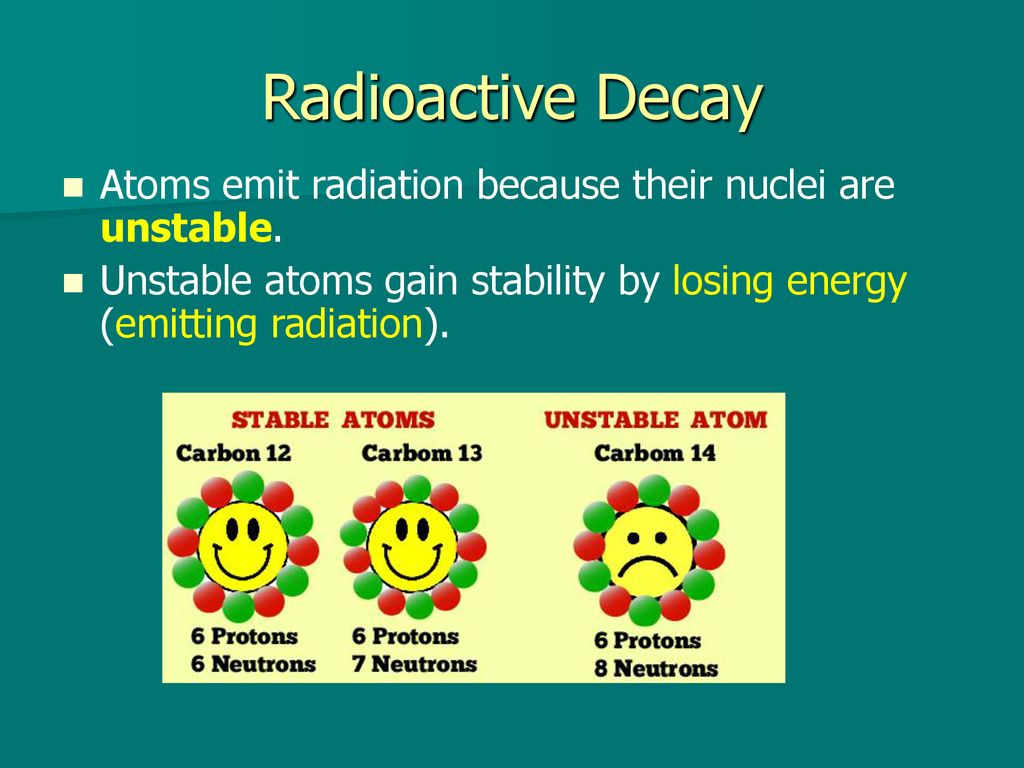
Atoms are considered unstable when they have not filled outer shell of electrons so atoms lose electron if their outer shell is filled by 12 or 3 electrons and atoms gain electrons when their. Radioactive atoms emit radiation because their nuclei are unstable. Explain how unstable atoms gain stability.
They undergo a series of radioactive decays until they reach a stable element State what quantities are conserved when balancing a nuclear reaction. Since it is losing energy it emits radiation in a process called radioactive decay. An atom gains stability by emitting radiation or a.
This activity helps students understand how emitting an alpha or beta particle changes the structure of an atom. Instability in atomic nuclei is causes by an excess of deficiency in the proton-to-neutron ratio. Valence electrons are the outermost electrons in an atom.
Unstable atoms gain stability by losing its energy. If the forces between the protons and the neutrons in the nucleus are unbalanced then the atom is unstable. I dont have the answer but I can tell you where to get it.
The protons stay together in the nucleus. The stability of atoms depends on their neutron-to-Protons Electrons proton ratio. The octet rule can be observed in the bonding between the carbon and oxygen atoms in a carbon dioxide molecule.
Recall that the number of protons in the nucleus is called the atomic. How do unstable atoms gain stability. Unstable atoms are also called radioactive Radioactive - Atoms which are energetically unstable and decay to a stable condition by emitting radiation are said to be radioactive.
So I know the basics an atom gains stability by having 8 electrons in its outer shell or 2 in some cases It can do this through covalent bonding or ionic bonding etc. In order to reach stability atoms will gain or lose electrons to achieve a full outermost energy level valence. Aug 5 2016 Atoms achieve stability in a single covalent bond by sharing valence electrons to create filled electron shells which are stable.
Her website should be shown. Radiation is emitted from atoms when an unstable atom decays to become more stable. The chicken is in stable equilibrium.
Answer 1 of 1. It ends up always in a place in which every direction is either uphill or flat so that gravity has nowhere to move it. In general the elements that obey this rule include the s-block elements and the p-block elements except hydrogen helium and lithium.
Ever wondered how atoms being almost empty manage to avoid crashing into each other. Here we have to look at two different stabilities of atoms. Stable atoms retain their form indefinitely while unstable atoms undergo radioactive decay.
An atom is stable because of a balanced nucleus that does not contain excess energy. Ive drawn it out. Ive been doing some questions and came across a few examples where an atom did not have 8 outershell electrons ie BCl3.
The molecules of the halogens oxygen nitrogen and carbon are known to obey the octet rule. Click on the link to her site and click on honors biology then online textbook. Unstable nuclei lose energy by emitting radiation in a spontaneous process called radioactive decay.
The main factor in determining whether an atom is stable or not is its ratio of neutrons to protons Create a table comparing the mass and charge of alpha beta and gamma radiation. Anna v Apr 10 2017 at 324 Add a comment 2. The nuclei that are to the left or to the right of the band of stability are unstable and exhibit radioactivity.
Atoms gain stability by reacting with other atoms means by forming compound. Mass number and atomic number. Discuss how radioactive atoms gain stability.
Unstable systems gain stability by losing energy. How does an unstable atom gain stability. 25 25 causing an atom to be radioactive.
Nuclear chemistry is the study of reactions that involve changes in nuclear structure. So Bohr imposed by postulates the stabilityThat is where the term stability comes from. Therefore the chicken is stable for front-to-back displacements as well as for side-to-side displacements.
Such unstable atoms rearrange their nuclei by emitting one or more forms of radiation which causes changes in the pn ratio that give greater stability. Basically it becomes stable when it runs out of downhill. Actoms and they will lose neutrons and protons as they attempt to become stable.
Co- means to share.
Nondestructive Evaluation Physics Atomic Elements

Why Does An Atom Want To Be Stable Quora
0 Comments